The Following Elements Were in the Required Reading
Chapter two. Atoms, Molecules, and Ions
2.five The Periodic Tabular array
Learning Objectives
Past the end of this section, you will be able to:
- State the periodic law and explain the system of elements in the periodic table
- Predict the full general properties of elements based on their location within the periodic table
- Identify metals, nonmetals, and metalloids by their backdrop and/or location on the periodic tabular array
As early chemists worked to purify ores and discovered more elements, they realized that diverse elements could be grouped together past their similar chemical behaviors. One such group includes lithium (Li), sodium (Na), and potassium (K): These elements all are shiny, comport heat and electricity well, and have similar chemical properties. A second grouping includes calcium (Ca), strontium (Sr), and barium (Ba), which besides are shiny, good conductors of heat and electricity, and have chemical properties in mutual. However, the specific properties of these two groupings are notably different from each other. For example: Li, Na, and Thou are much more reactive than are Ca, Sr, and Ba; Li, Na, and K grade compounds with oxygen in a ratio of two of their atoms to i oxygen atom, whereas Ca, Sr, and Ba form compounds with one of their atoms to one oxygen atom. Fluorine (F), chlorine (Cl), bromine (Br), and iodine (I) as well showroom similar properties to each other, simply these properties are drastically dissimilar from those of whatsoever of the elements above.
Dimitri Mendeleev in Russia (1869) and Lothar Meyer in Germany (1870) independently recognized that there was a periodic relationship among the properties of the elements known at that time. Both published tables with the elements arranged co-ordinate to increasing atomic mass. But Mendeleev went one step further than Meyer: He used his table to predict the existence of elements that would take the backdrop similar to aluminum and silicon, merely were withal unknown. The discoveries of gallium (1875) and germanium (1886) provided great support for Mendeleev'southward work. Although Mendeleev and Meyer had a long dispute over priority, Mendeleev'south contributions to the development of the periodic table are now more widely recognized (Figure 1).
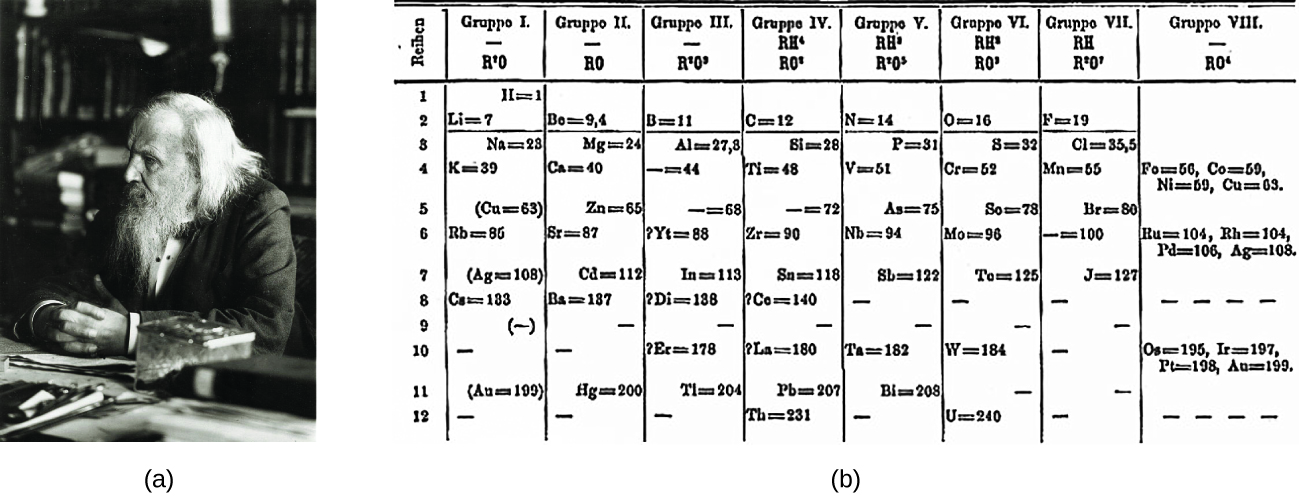
By the twentieth century, it became credible that the periodic human relationship involved atomic numbers rather than atomic masses. The mod statement of this relationship, the periodic constabulary, is as follows: the properties of the elements are periodic functions of their atomic numbers. A modern periodic table arranges the elements in increasing guild of their atomic numbers and groups atoms with similar properties in the same vertical column (Figure two). Each box represents an element and contains its diminutive number, symbol, average atomic mass, and (sometimes) name. The elements are bundled in vii horizontal rows, called periods or series, and eighteen vertical columns, called groups. Groups are labeled at the elevation of each column. In the United States, the labels traditionally were numerals with uppercase letters. Withal, IUPAC recommends that the numbers 1 through eighteen be used, and these labels are more mutual. For the table to fit on a single page, parts of two of the rows, a total of 14 columns, are usually written beneath the primary body of the table.
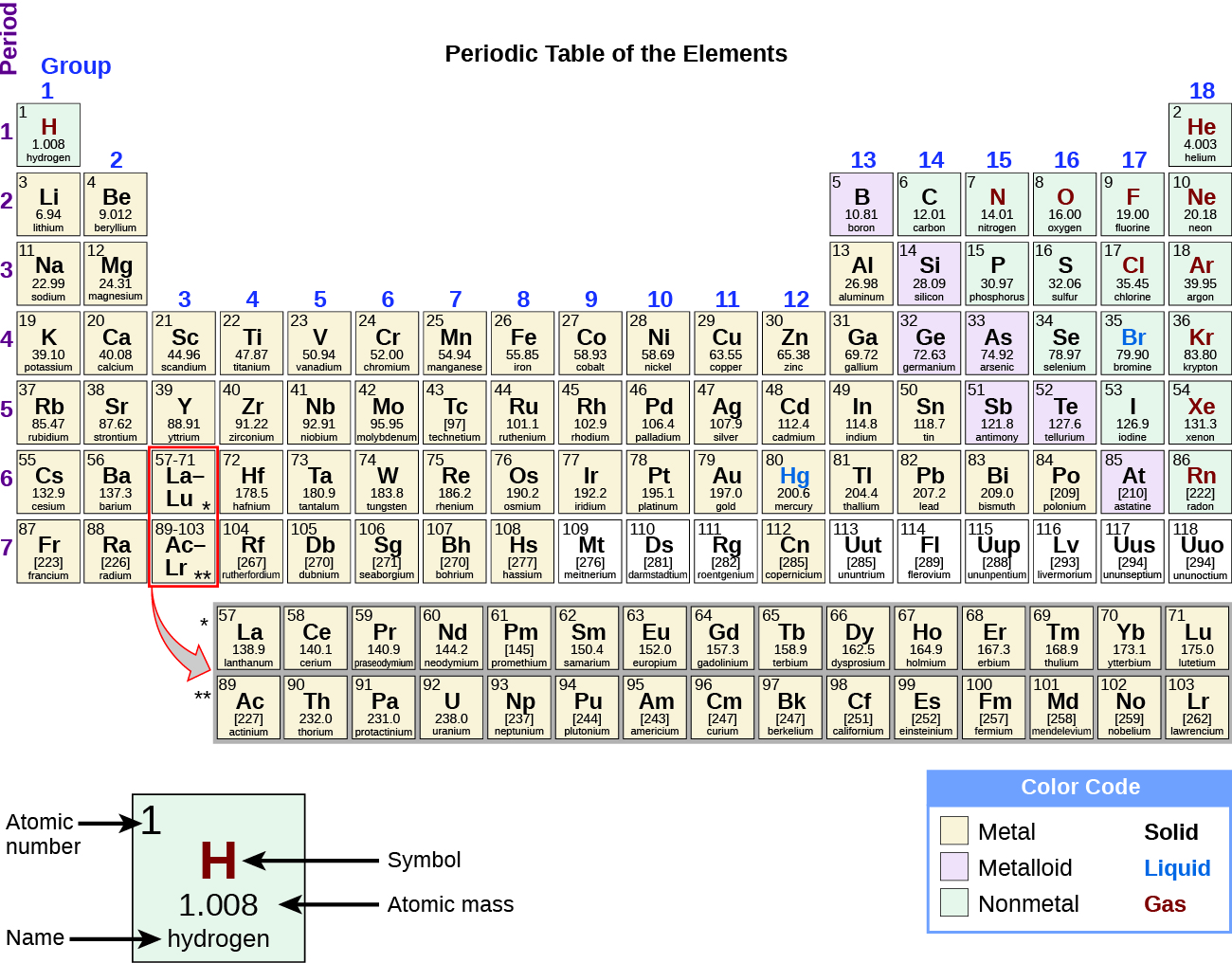
Many elements differ dramatically in their chemical and physical properties, merely some elements are similar in their behaviors. For case, many elements announced shiny, are malleable (able to be deformed without breaking) and ductile (can be drawn into wires), and carry heat and electricity well. Other elements are not shiny, malleable, or ductile, and are poor conductors of heat and electricity. We can sort the elements into big classes with mutual properties: metals (elements that are shiny, malleable, skillful conductors of heat and electricity—shaded yellow); nonmetals (elements that announced slow, poor conductors of estrus and electricity—shaded green); and metalloids (elements that conduct heat and electricity moderately well, and possess some properties of metals and some properties of nonmetals—shaded purple).
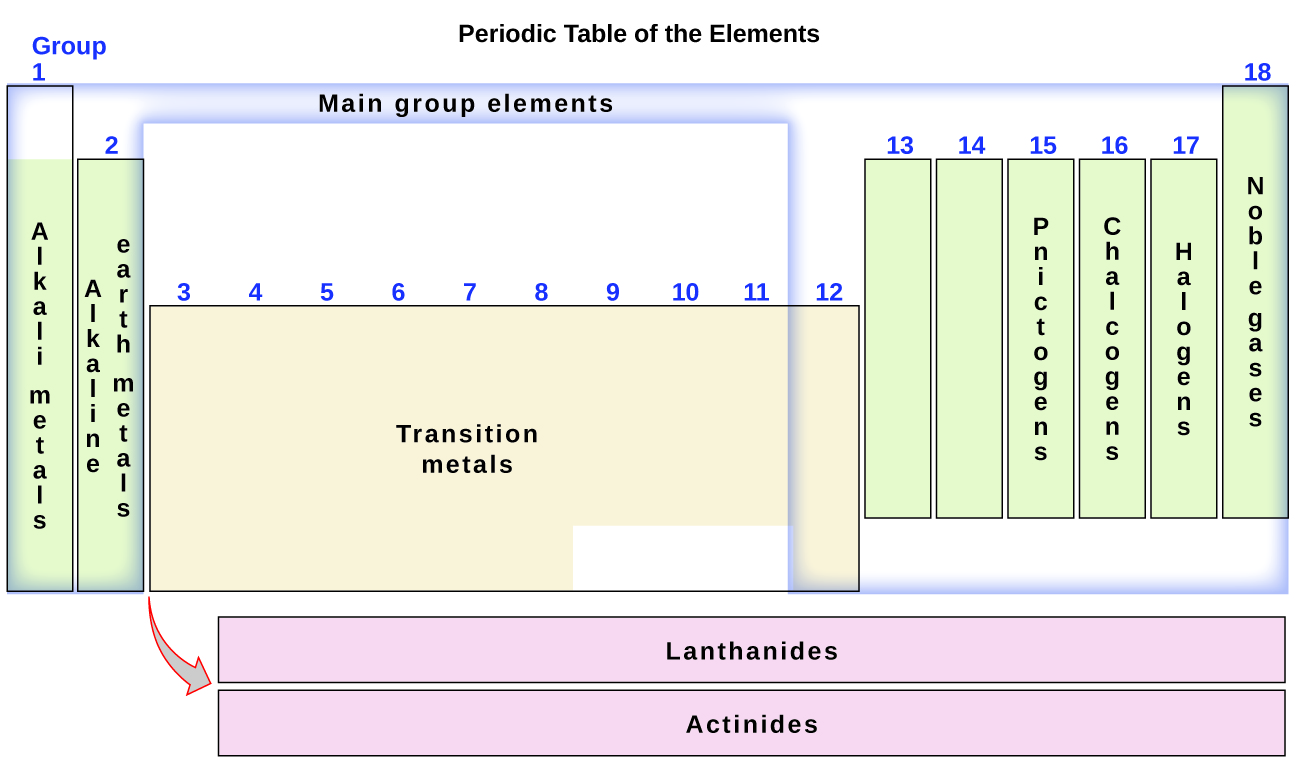
The elements can besides be classified into the main-grouping elements (or representative elements) in the columns labeled 1, 2, and xiii–18; the transition metals in the columns labeled 3–12; and inner transition metals in the two rows at the bottom of the table (the summit-row elements are called lanthanides and the bottom-row elements are actinides; Figure 3). The elements can be subdivided further past more specific properties, such equally the composition of the compounds they grade. For instance, the elements in group ane (the beginning cavalcade) course compounds that consist of one cantlet of the element and one cantlet of hydrogen. These elements (except hydrogen) are known as alkali metals, and they all have similar chemical properties. The elements in group 2 (the 2d column) course compounds consisting of one cantlet of the element and 2 atoms of hydrogen: These are called alkali metal earth metals, with similar properties amid members of that grouping. Other groups with specific names are the pnictogens (group 15), chalcogens (group 16), halogens (group 17), and the noble gases (grouping 18, as well known equally inert gases). The groups tin can also be referred to by the first element of the group: For example, the chalcogens tin exist called the oxygen group or oxygen family. Hydrogen is a unique, nonmetallic element with properties similar to both grouping 1A and group 7A elements. For that reason, hydrogen may be shown at the top of both groups, or by itself.

Click on this link for an interactive periodic table, which you can use to explore the properties of the elements (includes podcasts and videos of each chemical element). You may besides want to try this i that shows photos of all the elements.
Example 1
Naming Groups of Elements
Atoms of each of the following elements are essential for life. Give the group name for the post-obit elements:
(a) chlorine
(b) calcium
(c) sodium
(d) sulfur
Solution
The family names are as follows:
(a) halogen
(b) alkali metal earth metal
(c) alkali metal
(d) chalcogen
Check Your Learning
Give the group name for each of the following elements:
(a) krypton
(b) selenium
(c) barium
(d) lithium
Respond:
(a) noble gas; (b) chalcogen; (c) alkaline metal globe metal; (d) element of group i
In studying the periodic tabular array, you might take noticed something about the diminutive masses of some of the elements. Element 43 (technetium), element 61 (promethium), and well-nigh of the elements with atomic number 84 (polonium) and higher have their diminutive mass given in square brackets. This is done for elements that consist entirely of unstable, radioactive isotopes (yous will learn more than nigh radioactive decay in the nuclear chemistry affiliate). An boilerplate atomic weight cannot be adamant for these elements because their radioisotopes may vary significantly in relative abundance, depending on the source, or may not even be in nature. The number in foursquare brackets is the diminutive mass number (and approximate diminutive mass) of the most stable isotope of that element.
Key Concepts and Summary
The discovery of the periodic recurrence of like properties among the elements led to the formulation of the periodic tabular array, in which the elements are arranged in order of increasing atomic number in rows known every bit periods and columns known as groups. Elements in the aforementioned group of the periodic table have similar chemical properties. Elements can be classified as metals, metalloids, and nonmetals, or as a main-group elements, transition metals, and inner transition metals. Groups are numbered 1–xviii from left to right. The elements in grouping 1 are known as the alkali metals; those in grouping 2 are the alkaline earth metals; those in 15 are the pnictogens; those in 16 are the chalcogens; those in 17 are the halogens; and those in eighteen are the noble gases.
Chemical science End of Affiliate Exercises
- Using the periodic table, classify each of the following elements as a metal or a nonmetal, and so further classify each every bit a main-group (representative) element, transition metal, or inner transition element:
(a) uranium
(b) bromine
(c) strontium
(d) neon
(e) gold
(f) americium
(one thousand) rhodium
(h) sulfur
(i) carbon
(j) potassium
- Using the periodic table, classify each of the post-obit elements as a metal or a nonmetal, and then further allocate each as a master-group (representative) element, transition metal, or inner transition metal:
(a) cobalt
(b) europium
(c) iodine
(d) indium
(e) lithium
(f) oxygen
(h) cadmium
(i) terbium
(j) rhenium
- Using the periodic table, identify the lightest member of each of the following groups:
(a) noble gases
(b) element of group i earth metals
(c) brine metals
(d) chalcogens
- Using the periodic table, identify the heaviest member of each of the following groups:
(a) alkali metals
(b) chalcogens
(c) noble gases
(d) alkaline globe metals
- Utilise the periodic table to give the proper noun and symbol for each of the following elements:
(a) the noble gas in the same period as germanium
(b) the element of group ii in the same period every bit selenium
(c) the halogen in the same menstruation as lithium
(d) the chalcogen in the same menses as cadmium
- Utilize the periodic table to give the proper name and symbol for each of the post-obit elements:>
(a) the halogen in the same period every bit the alkaline with xi protons
(b) the alkali metal earth metallic in the same period with the neutral element of group 0 with 18 electrons
(c) the noble gas in the same row equally an isotope with 30 neutrons and 25 protons
(d) the noble gas in the same period as aureate
- Write a symbol for each of the following neutral isotopes. Include the atomic number and mass number for each.
(a) the alkali metal with 11 protons and a mass number of 23
(b) the element of group 0 element with 75 neutrons in its nucleus and 54 electrons in the neutral atom
(c) the isotope with 33 protons and forty neutrons in its nucleus
(d) the element of group ii with 88 electrons and 138 neutrons
- Write a symbol for each of the post-obit neutral isotopes. Include the diminutive number and mass number for each.
(a) the chalcogen with a mass number of 125
(b) the halogen whose longest-lived isotope is radioactive
(c) the noble gas, used in lighting, with x electrons and 10 neutrons
(d) the lightest alkali metal with three neutrons
Glossary
- actinide
- inner transition metal in the bottom of the lesser two rows of the periodic table
- alkaline metal
- chemical element in group one
- alkaline earth metal
- element in group 2
- chalcogen
- element in group 16
- group
- vertical column of the periodic table
- halogen
- element in grouping 17
- inert gas
- (also, noble gas) chemical element in group 18
- inner transition metal
- (too, lanthanide or actinide) element in the bottom 2 rows; if in the first row, likewise called lanthanide, or if in the 2d row, also called actinide
- lanthanide
- inner transition element in the top of the bottom two rows of the periodic table
- main-group element
- (as well, representative element) element in columns 1, 2, and 12–18
- metal
- element that is shiny, malleable, skillful usher of estrus and electricity
- metalloid
- element that conducts heat and electricity moderately well, and possesses some backdrop of metals and some properties of nonmetals
- noble gas
- (also, inert gas) element in group 18
- nonmetal
- chemical element that appears dull, poor usher of heat and electricity
- menses
- (likewise, series) horizontal row of the periodic table
- periodic law
- properties of the elements are periodic function of their atomic numbers.
- periodic table
- table of the elements that places elements with like chemic properties close together
- pnictogen
- element in group 15
- representative element
- (also, main-group element) element in columns ane, ii, and 12–18
- serial
- (too, menstruum) horizontal row of the period tabular array
- transition metal
- element in columns 3–eleven
Solutions
Answers to Chemistry End of Chapter Exercises
1. (a) metal, inner transition metallic; (b) nonmetal, representative element; (c) metal, representative element; (d) nonmetal, representative element; (east) metal, transition element; (f) metal, inner transition metallic; (g) metal, transition metal; (h) nonmetal, representative element; (i) nonmetal, representative element; (j) metal, representative chemical element
three. (a) He; (b) Exist; (c) Li; (d) O
5. (a) krypton, Kr; (b) calcium, Ca; (c) fluorine, F; (d) tellurium, Te
7. (a) [latex]_{11}^{23}\text{Na}[/latex]; (b) [latex]_{54}^{129}\text{Xe}[/latex]; (c) [latex]_{33}^{73}\text{Equally}[/latex] ; (d) [latex]_{88}^{226}\text{Ra}[/latex];
Source: https://opentextbc.ca/chemistry/chapter/2-5-the-periodic-table/
Belum ada Komentar untuk "The Following Elements Were in the Required Reading"
Posting Komentar